Which Statement Describes Cloning Animals By The Nuclear Transfer Technique?
- Review
- Open up Access
- Published:
Cloning animals by somatic prison cell nuclear transfer – biological factors
Reproductive Biology and Endocrinology volume i, Article number:98 (2003) Cite this article
Abstract
Cloning by nuclear transfer using mammalian somatic cells has enormous potential awarding. However, somatic cloning has been inefficient in all species in which live clones have been produced. High abortion and fetal mortality rates are commonly observed. These developmental defects accept been attributed to incomplete reprogramming of the somatic nuclei by the cloning procedure. Various strategies accept been used to amend the efficiency of nuclear transfer, however, meaning breakthroughs are withal to happen. In this review we will discuss studies conducted, in our laboratories and those of others, to proceeds a better understanding of nuclear reprogramming. Because cattle are a species widely used for nuclear transfer studies, and more laboratories have succeeded in cloning cattle than any other specie, this review volition be focused on somatic prison cell cloning of cattle.
Introduction
Somatic jail cell cloning (cloning or nuclear transfer) is a technique in which the nucleus (DNA) of a somatic cell is transferred into an enucleated metaphase-Two oocyte for the generation of a new individual, genetically identical to the somatic prison cell donor (Figure ane). The success of cloning an unabridged animal, Dolly, from a differentiated developed mammary epithelial cell [1] has created a revolution in science. It demonstrated that genes inactivated during tissue differentiation tin be completely re-activated by a process called nuclear reprogramming: the reversion of a differentiated nucleus back to a totipotent status. Somatic cloning may be used to generate multiple copies of genetically elite farm animals, to produce transgenic animals for pharmaceutical poly peptide production or xeno-transplantation [2–v], or to preserve endangered species. With optimization, it besides promises enormous biomedical potential for therapeutic cloning and allo-transplantation [6]. In addition to its practical applications, cloning has go an essential tool for studying gene function [7], genomic imprinting [8], genomic re-programming [nine–12], regulation of evolution, genetic diseases, and factor therapy, as well every bit many other topics.
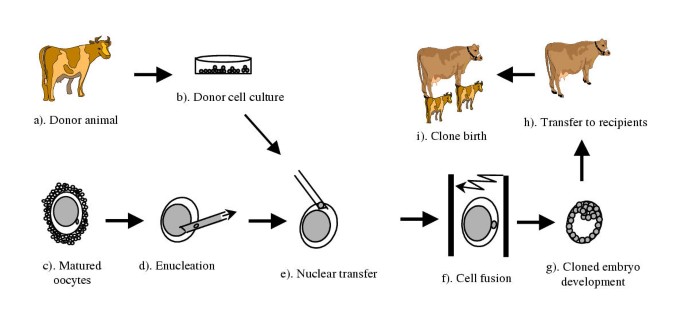
Schematic diagram of the somatic cloning process. Cells are collected from donor (a) and cultured in vitro (b). A matured oocyte (c) is and then enucleated (d) and a donor cell is transferred into the enucleated oocyte (e). The somatic jail cell and the oocyte is then fused (f) and the embryos is allowed to develop to a blastocyst in vitro (g). The blastocyst tin and so be transferred to a recipient (h) and cloned animals are born after completion of gestation (i).
One of the almost difficult challenges faced, yet, is cloning'due south depression efficiency and high incidence of developmental abnormalities [13–nineteen]. Currently, the efficiency for nuclear transfer is betwixt 0–x%, i.e., 0–10 live births after transfer of 100 cloned embryos. Developmental defects, including abnormalities in cloned fetuses and placentas, in addition to loftier rates of pregnancy loss and neonatal decease take been encountered by every research team studying somatic cloning. It has been proposed that low cloning efficiency may be largely attributed to the incomplete reprogramming of epigenetic signals [20–23].
Factors affecting nuclear reprogramming
Various strategies have been employed to modify donor cells and the nuclear transfer procedure in attempts to improve the efficiency of nuclear transfer. Almost of these efforts are focused on donor cells. These include: a) synchrony of the jail cell cycle stage of donor cells [24–26], as well as synchrony betwixt donor cells and recipient oocytes [27, 28]; b) using somatic cells from donors of diverse ages [29–33], tissue origins [26, 34–39], passages [16, 40, 41] and civilisation conditions [42]; c) transfer of stem cells with low levels of epigenetic marks [43–48]; and d) modifying epigenetic marks of donor cells with drugs [49–51]. Although the efficiency of nuclear transfer has been dramatically improved from the initial success rate of one live clone born from 277 embryo transfers [1], none of the aforementioned efforts abolished the common bug associated with nuclear transfer. These observations suggest that further studies on nuclear reprogramming are needed in order to understand the underlying mechanisms of reprogramming and significantly ameliorate the ability of the differentiated somatic nuclei to be reprogrammed. In the following section, we will discuss several strategies used to meliorate nuclear transfer efficiencies.
Serum starvation of donor cells
Serum starvation was used in the creation of Dolly and was believed essential to the success of nuclear transfer [1]. Serum starvation induces quiescence of cultured cells, and arrests them at the cell cycle stage of G0. Most laboratories that take succeeded with nuclear transfer take utilized a serum starvation handling. However, at that place is a argue equally to whether inducing quiescence is required for successful nuclear transfer. Cibelli et al. [52] proposed that G0 was unnecessary and that calves could be produced from cycling cells. In his study, actively dividing bovine fibroblasts were used for nuclear transfer and iv calves were born from 28 embryos transferred to xi recipients. Because 56% of cycling cells in that written report were in G1 stage, information technology is likely that all cloned animals produced in this written report were from donor cells at G1 stage. Cells at G2, South or M would not be expected to generate cloned animals in this study because they are incompatible with the recipient oocytes used. This report demonstrated that cells at G1 stage can produce live cloned animals and G0 induction is non essential.
Since the report of Cibelli and colleagues, many laboratories take compared nuclear transfer using donor cells with and without serum starvation. In our study, we used cells from a 17-yr one-time male Japanese Black beefiness bull and found that serum starvation was not required for successful cloning because cloned embryos and animals were produced from cells non subjected to serum starvation (Tabular array 1) [sixteen]. Furthermore, serum starvation did not take a benign outcome on the blastocyst development of cloned embryos.
In other studies in which serum starvation vs. no starvation were directly compared, evidence was found that both quiescent and proliferating somatic donor cells can exist fully reprogrammed later on nuclear transfer and result in viable offspring [25, 26, 29, 53, 54]. However, information technology is yet debatable which cell cycle phase, G0 or G1, upshot in the best cloning efficiency. Interestingly, Zechkerchenko et al. [53] observed a positive upshot of serum starvation on the efficiency of nuclear transfer using bovine fetal fibroblasts. Although Cho et al. [55] did not observe an comeback in blastocyst rate from any of four dissimilar cell types (cumulus, fibroblast, uterine and oviduct epithelial cells). Similar observations were noted by Hills et al. [29] who reported that serum starvation of adult donor cells did not improve evolution rates of cloned embryos to blastocyst, but when fetal cells were serum-starved, at that place was a pregnant increase in their blastocyst evolution. Conversely, Rho et al. [54] found that fetal transgenic lines were not different in blastocyst development with or without serum starvation or confluency.
Recently, Kasinathan et al. [25] evaluated methods for generating G0 and G1 cell populations and compared their development following cloning. They found that a high degree of confluence was more effective than serum starvation for arresting cells in G0, and G1 cells could be obtained using a "milkshake-off" procedure. In this study, no differences in in vitro development were observed between embryos derived from the loftier-confluence cells (G0) or from the "shaken-off" cells (G1). Nevertheless, when embryos from each handling were transferred into 50 recipients, v calves (10% of embryos transferred) were obtained from embryos derived from the "shake-off" cells, whereas no embryos from the confluent cells survived beyond 180 days of gestation. Kasinathan et al. [25] concluded that nuclear transfer donor prison cell cycle stage is important, particularly effecting belatedly fetal evolution, and that actively dividing G1 cells back up higher evolution rates than cells in G0. Despite the fact that Kasinathan's report did not produce alive clones from G0 cells, a high nuclear transfer success rate was obtained past Cho et al. [55] who subjected donor cells to serum starvation and found no improvement in blastocyst evolution from developed donor cells, but resulted in a 27.3% calving rate.
To further complicate the matter, Wells et al. [26] compared two unlike types of non-transfected bovine fetal fibroblasts (BFFs) that were synchronized in G0, G1 or different phases within G1. They showed that serum starvation into G0 resulted in a significantly higher percentage of viable calves at term than did synchronization in early G1 or late G1. For transgenic fibroblasts, however, cells selected in G1 showed significantly higher development to term of calves and higher post-natal survival to weaning, than cells in G0. They suggest that it may exist necessary to coordinate donor cell type and cell wheel stage to maximize overall cloning efficiency.
In summary, it is articulate that quiescence is not necessary for the success of nuclear transfer because cells non subjected to serum starvation tin also produce live clones. Even so, information technology remains unclear which cell cycle stage, G0 or G1, imparts a higher nuclear transfer efficiency. This question will continue to be debated until large-scale nuclear transfer studies can be conducted.
Cloning competence of various somatic cell types
Many somatic jail cell types, including mammary epithelial cells, ovarian cumulus cells, fibroblast cells from pare and internal organs, various internal organ cells, Sertoli cells [38, 56], macrophage [56] and blood leukocytes [34, 35] have been successfully utilized for nuclear transfer. A clear consensus, however, has not yet been reached as to the superior somatic cell type for nuclear transfer. This is due in part to the fact that dissimilar laboratories employ various procedures; and cell culture, nuclear transfer, and micromanipulation all crave critical technical skills. In guild to brand these comparisons valid, the procedures and techniques used, equally well every bit the skill of lab personnel, must be identical for each donor animal and prison cell blazon. To compare the competence of different jail cell types for reprogramming by cloning, we avoided animal variation past looking at the cloning competence of three cell types: ovarian cumulus, mammary epithelial and pare fibroblast cells, all from the same donor animal, a xiii-year-erstwhile elite diary moo-cow.
The power of donor cells to be reprogrammed was assessed by the evolution of cloned embryos in vitro and by the nativity of cloned calves following embryo transfer. Equally shown in Tables two and 3, although no differences were detected in the cleavage rates of embryos from three different jail cell types, cumulus cells produced the highest charge per unit of blastocyst evolution in this report and resulted in half-dozen total-term cloned calves. Furthermore, four out of the six calves derived from cumulus cells survived and were withal healthy at nearly 4 years of age (Table 3). In contrast, the poorest in vitro development, and no full-term survival, was obtained with mammary epithelial cells. Skin fibroblast cells resulted in an intermediate rate of in vitro development and gave rise to four full-term cloned calves.
Our results showed that the donor cell type can significantly touch embryo development in vitro too every bit in vivo. Cumulus cells proved to be the near effective prison cell type for somatic cloning according to both the in vitro development test as well every bit full-term survival. These results suggest that DNA from cumulus cells is more effectively reprogrammed following nuclear transfer. Our results agreed with those obtained in mice [57] where they compared the nuclear transfer efficiency of neuronal, Sertoli and cumulus cells, and obtained the best live birth rate from cumulus prison cell-derived cloned embryos. Furthermore, information technology was reported that cumulus cell-derived cloned mice exercise not have widespread dysregulation of imprinting [23]. Kato et al. [15, 36] compared cells from the liver, testis, skin, ear, along with cumulus and oviductal cells and concluded that cumulus and oviduct epithelial cells are the most suitable for nuclear donors. Evidence supporting the superiority of cumulus cells for nuclear transfer likewise comes from the written report of Forsberg et al. [58] who conducted large numbers of embryo transfer in cattle. It was shown that cumulus cells gave an overall 15.2% calving rate, while fetal genital ridge cells, and fibroblast cells produced a 9% calving rate. Adult fibroblast cells, in this study, gave the lowest calving rate of only 5%.
In summary, among the somatic cell types tested, the consensus from numerous laboratories is that cumulus cells give the highest cloning efficiency and event in the least number of abnormalities in cloned animals.
Outcome of donor historic period
By using a pattern similar to the donor prison cell blazon comparison, we studied the cloning efficiency of fibroblast cells from donors of different ages. We found that cells from fetuses and newborn animals were more efficient in nuclear transfer. Nonetheless, when cells from adult animals were used, little changes were observed in the cloning efficiency of cells from cattle varying in historic period from 2 to16-years-old (Table 4).
Similarly, Renard et al. [31], Hills et al. [29] and Wakayama and Yanagimachi [56] also reported that development rates of somatic cloned embryo remained similar regardless of donor age. Nonetheless, Kato et al. [36] noted that clones derived from developed cells frequently aborted in the later stages of pregnancy, and calves developing to term showed a higher number of abnormalities than did those derived from newborn or fetal cells. Forsberg et al. [58] transferred a large number of cloned embryos in cattle. They besides concluded that, in general, embryos cloned from fetal cells produced higher pregnancy and calving rates than those from adult cells.
In conclusion, it appears that cells from fetuses, as well as aged adults, can pb to comparable blastocyst development of cloned embryos. Notwithstanding, fetal cells may be better than adult cells in producing salubrious live births. This might be due to the fact that the somatic cells of adult animals have accumulated more genetic mutations/are more terminally differentiated than fetal cells, and are thus more probable to neglect at total term evolution.
Upshot of cell culture duration (passage numbers)
Our group was the first to directly compare passage effect of donor cells on the outcome of nuclear transfer [xvi]. In our written report, we found that cells of afterwards passages (upwardly to fifteen) could also support clone development to full term (Tabular array 5).
Comparable to our findings were those of Arat et al. [40] who established a chief jail cell line from granulosa cells and transfected them with the green fluorescence protein (GFP) factor. Not-transfected cells were used for cloning betwixt passage 10 and xv as either serum-starved or serum-fed donor cells. There were no differences in development to the blastocyst stage for nuclear transfer embryos from transfected or non-transfected or from serum-starved or serum-fed cells. Blastocyst development rates of embryos produced from donor cells at passage 15, notwithstanding, were significantly higher than those produced with cells at passage 10, 11, and 13. Developmental competence of later passages, up to 16 [54] and as loftier as 36, from fibroblast from a cloned fetus [41], have also been reported.
The demonstration that later passages can support clone development is essential for utilizing somatic cloning for gene-knockout studies, in which single cells must be clonally expanded to generate sufficient cells for nuclear transfer [7]. These afore-mentioned studies advise that cells of higher passages were receptive to nuclear reprogramming. Additional back up for this hypothesis comes from a contempo written report by Enright et al. [59] who showed that cells of afterwards passages incorporate less epigenetic modifications, i.east., their histones are more acetylated than in earlier passages. This ascertainment agrees with an earlier notion that in vitro civilization of cells can induce expression of genes that were not expressed before civilization [60, 61]. Furthermore, Hills et al. [62] reported that a greater proportion of belatedly passage cells (passage 18), vs. earlier passage cells (passage two), were found to be in G0/G1 whether or not they were in serum-starved culture conditions.
Effect of modification of pre-existing epigenetic marks in donor cells
Histone acetylation and Deoxyribonucleic acid methylation are heritable modifications of the chromatin that practise non involve changes in gene sequences (epigenetic signals). These epigenetic modifications are believed responsible for the derivation of various cell types with the same genetic makeup. In natural reproduction, relatively low levels of DNA methylation exist in the gametes, which are farther de-methylated during early embryo development [63, 64]. With nuclear transplantation, the somatic donor nucleus carries the specific epigenetic modifications of its tissue type, which must be erased during nuclear reprogramming. Therefore, the levels of epigenetic modification existing in donor cells may affect their reprogrammability following nuclear transfer. As discussed before, a discrepancy in the donor jail cell'south susceptibility to reprogramming has been observed between different prison cell types, resulting in differences in vitro and in vivo development of cloned embryos. Therefore, treating donor cells with pharmacological agents to remove some epigenetic marks prior to nuclear transfer may ameliorate the power of the donor cells to be fully reprogrammed by the recipient karyoplast.
Two reagents take been widely used for the alteration of the levels of epigenetic modification of somatic cells. Trichostatin A (TSA) and 5-aza-deoxy-cytadine (5-aza-dC) have been found to increase histone acetylation and decrease DNA methylation, respectively. These changes accept been associated with increases of gene expression. Recently, we conducted studies in which the pre-existing epigenetic marks in donor cells were reduced by these drugs [49]. We found that global epigenetic marks in donor cells can be modified by treatment with TSA or 5-aza-dC. Unfortunately, treating donor cells with 5-aza-dC reduced blastocyst formation of cloned embryos. Previously, Jones et al. [fifty] and Zhou et al. [51] treated bovine fetal fibroblast cells and mouse stem cells with much higher doses of 5-aza-C (1 or 5 μm) and also found that blastocyst development of cloned embryos were reduced. The consensus from these studies [49–51] suggests that lowering the levels of Deoxyribonucleic acid methylation in donor cells does non always improve evolution of cloned embryos. At high concentrations, five-aza-dC may have been cytotoxic to the donor cells. Additionally, prolonged treatment at a lower concentration, as was the example in our written report, may accept caused severe hypo-methylation, and resulted in disrupted expression of essential genes important for embryo development. Therefore, further experiments are required to test the effects of lower concentrations and shorter durations of 5-aza-dC handling on donor cells.
Treating donor cells with TSA, by contrast, significantly improved development of cloned embryos. Previous reports indicated that treatment of mouse stem cells with TSA reduced evolution of cloned embryos [51]. The differences between these findings may be due to the variation in the concentrations of TSA used. Prior to nuclear transfer, we treated donor cells with a wide range of TSA concentrations and identified the lowest concentration capable of inducing histone hyperacetylation (1.25 μM). The lowest concentration tested (0.08 μM), did not crusade hyperacetylation, but resulted in appreciable changes in cell morphology, similar to those described previously [65]. Information technology was this lower concentration of TSA (0.08 μM) that improved evolution of cloned embryos in our written report, while the higher concentration (ane.25 μM) inhibited embryo development. The detrimental effect of a higher dose of TSA on embryo development may be explained by the fact that treatment of cells with loftier concentrations of TSA causes chromatin breaks and apoptosis [66].
Decision
Somatic jail cell cloning by nuclear transfer is a relatively new technology with many potential applications. Still, at the current stage of development, the reprogramming of epigenetic inheritance by nuclear transfer is still incomplete. Further efforts and new paradigms are needed to perfect this applied science and extend it to its fullest potential.
References
-
Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KHS: Viable offspring derived from fetal and adult mammalian cells. Nature. 1997, 385: 810-813. 10.1038/385810a0.
-
Anderson GB, Seidel GE: Cloning for profit. Science. 1998, 280: 1400-1401. x.1126/science.280.5368.1400.
-
Polejaeva IA, Campbell KHS: New advances in somatic cell nuclear transfer: Awarding in transgenesis. Theriogenology. 2000, 53: 117-126. 10.1016/S0093-691X(99)00245-9.
-
Robl J: Development and awarding of technology for big scale cloning of cattle. Theriogenology. 1999, 51: 499-508. 10.1016/S0093-691X(98)00243-X.
-
Stice SL, Robl JM, Ponce de Leon FA, Jerry J, Golueke PG, Cibelli JB, Kane JJ: Cloning: new breakthroughs leading to commercial opportunities. Theriogenology. 1998, 49: 129-138. x.1016/S0093-691X(97)00407-X.
-
Lanza RP, Cibelli JB, West Physician: Homo therapeutic cloning. Nat Med. 1999, 5: 975-977. 10.1038/12404.
-
Capecchi MR: How shut are we to implementing gene targeting in animals other than the mouse?. Proc Natl Acad Sci The states. 2000, 97: 956-957. 10.1073/pnas.97.3.956.
-
Solter D: Imprinting. Int J Dev Biol. 1998, 42: 951-954.
-
De Sousa PA, Winger Q, Hill JR, Jones K, Watson AJ, Westhusin ME: Reprogramming of fibroblast nuclei subsequently transfer into bovine oocytes. Cloning. 1999, 1: 63-69. 10.1089/15204559950020102.
-
Munsie MJ, Michalska AE, O'Brien CM, Trounson AO, Pera MF, Mountford PS: Isolation of pluripotent embryonic stem cells from reprogrammed adult mouse somatic nuclei. Curr Biol. 2000, 10: 989-992. 10.1016/S0960-9822(00)00648-five.
-
Surani MA: Reprogramming of genome role through epigenetic inheritance. Nature. 2001, 414: 122-128. 10.1038/35102186.
-
Winger QA, Hill JR, Shin T, Watson AJ, Kraemer DC, Westhusin ME: Genetic reprogramming of lactate dehydrogenase, citrate synthase, and phosphofructokinase mRNA in bovine nuclear transfer embryos produced using bovine fibroblast cell nuclei. Mol Reprod Dev. 2000, 56: 458-464. 10.1002/1098-2795(200008)56:4<458::Assistance-MRD3>iii.iii.CO;2-C.
-
Garry FB, Adams R, McCann JP, Odde KG: Postnatal characteristics of calves produced by nuclear transfer cloning. Theriogenology. 1996, 45: 141-152. 10.1016/0093-691X(95)00363-D.
-
Hill JR, Roussel AJ, Cibelli JB, Edwards JF, Hooper NL, Miller MW, Thompson JA, Looney CR, Westhusin ME, Robl JM, Stice SL: Clinical and pathologic features of cloned transgenic calves and fetuses (13 instance studies). Theriogenology. 1999, 51: 1451-1465. x.1016/S0093-691X(99)00089-viii.
-
Kato Y, Tani T, Sotomaru Y, Kurokawa Grand, Kato J, Doguchi H, Yasue H, Tsunoda Y: Eight calves cloned from somatic cells of a single adult. Science. 1998, 282: 2095-2098. 10.1126/science.282.5396.2095.
-
Kubota C, Yamakuchi H, Todoroki J, Mizoshita K, Tabara N, Hairdresser G, Yang X: Half dozen cloned calves produced from adult fibroblast cells after long-term culture. Proc Natl Acad Sci U.s.. 2000, 97: 990-995. ten.1073/pnas.97.iii.990.
-
Renard J-P, Chastnat S, Chesne P, Richard C, Marchal J, Cordonnier N, Chavatte P, Vignon X: Lymphoid hypoplasia and somatic cloning. Lancet. 1999, 353: 1489-1491. 10.1016/S0140-6736(98)12173-6.
-
Walker SK, Hartwich KM, Seamark RF: The product of unusually large offspring following embryo manipulation: concepts and changes. Theriogenology. 1996, 45: 111-120. 10.1016/0093-691X(95)00360-Chiliad.
-
Young LE, Sinclear KD, Wilmut I: Large offspring syndrome in cattle and sheep. Rev Reprod. 1998, 3: 155-163. ten.1530/revreprod/3.3.155.
-
Bourc'his D, Le Bourhis D, Patin D, Niveleau A, Comizzoli P, Renard J-P, Viegas-Pe'quignot E: Delayed and incomplete reprogramming of chromosome methylation patterns in bovine cloned embryos. Curr Biol. 2001, xi: 1542-1546. 10.1016/S0960-9822(01)00480-8.
-
Dean W, Santos F, Stojkovic Grand, Zakhartchenko 5, Walter J, Wolf E, Reik Due west: Conservation of methylation reprogramming in mammalian development: abnormal reprogramming in cloned embryos. Proc Natl Acad Sci USA. 2001, 98: 13734-13738. 10.1073/pnas.241522698.
-
Kang Y-K, Koo D-B, Park J-S, Choi Y-H, Lee K-Yard, Han Y-1000: Aberrant methylation of donor genome in cloned bovine embryos. Nat Genet. 2001, 28: 173-177. 10.1038/88903.
-
Rideout WM, Eggan K, Jaenisch R: Nuclear cloning and epigenetic reprogramming of the genome. Science. 2001, 293: 1093-1098. 10.1126/science.1063206.
-
Gibbons J, Arat South, Rzucidlo J, Miyoshi K, Waltenburg R, Respess D, Venable A, Stice Due south: Enhanced survivability of cloned calves derived from roscovitine-treated adult somatic cells. Biol Reprod. 2002, 66: 895-900.
-
Kasinathan P, Knott JG, Wang Z, Jerry DJ, Robl JM: Production of calves from G1 fibroblasts. Nat Biotechnol. 2001, 19: 1176-1178. 10.1038/nbt1201-1176.
-
Wells DN, Laible G, Tucker FC, Miller AL, Oliver JE, Xiang T, Forsyth JT, Berg MC, Cockrem K, L'Huillier PJ, Tervit 60 minutes, Oback B: Coordination between donor cell blazon and jail cell cycle stage improves nuclear cloning efficiency in cattle. Theriogenology. 2003, 59: 45-59. ten.1016/S0093-691X(02)01273-6.
-
Campbell KH, Loi P, Cappai P, Wilmut I: Improved development to blastocyst of ovine nuclear transfer embryos reconstructed during the presumptive S-phase of enucleated activated oocytes. Biol Reprod. 1994, 50: 1385-1393.
-
Du F, Sung L-Y, Tian 90, Yang X: Differential Cytoplast Requirement for Embryonic and Somatic Prison cell Nuclear Transfer in Cattle. Mol Reprod Dev. 2002, 63: 183-191. 10.1002/mrd.10172.
-
Hill JR, Winger QA, Long CR, Looney CR, Thompson JA, Westhusin ME: Evolution rates of male bovine nuclear transfer embryos derived from adult and fetal cells. Biol Reprod. 2000, 62: 1135-1140.
-
Kasinathan P, Knott JG, Moreira PN, Burnside AS, Jerry DJ, Robl JM: Effect of fibroblast donor cell age and prison cell wheel on evolution of bovine nuclear transfer embryos in vitro. Biol Reprod. 2001, 64: 1487-1493.
-
Renard JP: Chromatin remodeling and potential for full term development of cloned embryos. In Proceedings of Transgenic Animals in Enquiry. Conference Proceedings of Transgenic Animal Inquiry Briefing: Aug, 1999. 1999, Tahoe City, CA, 15-
-
Tian XC, Xu J, Yang 10: Normal telomere lengths found in cloned cattle. Nat Genet. 2000, 26: 272-273. 10.1038/81559.
-
Xue F, Tian XC, Kubota C, Du F, Taneja M, Dinnyes A, Dai Y, Levine H, Pereira LV, Yang X: Aberrant 10-Chromosome inactivation in deceased cattle derived from somatic cloning. Nat Genet. 2002, 31: 216-220. 10.1038/ng900.
-
Galli C, Duchi R, Moor RM, Lazzari Thousand: Mammalian leukocytes comprise all the genetic information necessary for the development of a new individual. Cloning. 1999, ane: 161-170. ten.1089/15204559950019924.
-
Hochedlinger Thou, Jaenisch R: Monoclonal mice generated by nuclear transfer from mature B and T donor cells. Nature. 2002, 415: 1035-1038. ten.1038/nature718.
-
Kato Y, Tani T, Tsunoda Y: Cloning of calves from various somatic jail cell types of male and female person adult, newborn and fetal cows. J Reprod Fertil. 2000, 120: 231-237. ten.1530/reprod/120.two.231.
-
Miyashita N, Shiga K, Yonai G, Kaneyama K, Kobayashi Southward, Kojima T, Goto Y, Kishi 1000, Aso H, Suzuki T, Sakaguchi M, Nagai T: Remarkable differences in telomere lengths among cloned cattle derived from unlike cell types. Biol Reprod. 2002, 66: 1649-1655.
-
Ogura A, Inoue K, Ogonuki Due north, Noguchi A, Takano K, Nagano R, Suzuki O, Lee J, Ishino F, Matsuda J: Production of male cloned mice from fresh, cultured, and cryopreserved immature Sertoli cells. Biol Reprod. 2000, 62: 1579-1584.
-
Shiga Yard, Fujita T, Hirose Thou, Sasae Y, Nagai T: Production of calves past transfer of nuclei from cultured somatic cells obtained from Japanese black bulls. Theriogenology. 1999, 52: 527-535. 10.1016/S0093-691X(99)00149-1.
-
Arat South, Rzucidlo SJ, Gibbons J, Miyoshi K, Stice SL: Production of transgenic bovine embryos by transfer of transfected granulosa cells into enucleated oocytes. Mol Reprod Dev. 2001, threescore: 20-26. 10.1002/mrd.1057.
-
Liu L, Shin T, Pryor JH, Kraemer D, Westhusin M: Regenerated bovine fetal fibroblasts support loftier blastocyst development post-obit nuclear transfer. Cloning. 2001, 3: 51-58. 10.1089/15204550152475554.
-
Zakhartchenko V, Alberio R, Stojkovic M, Prelle 1000, Schernthaner Due west, Stojkovic P, Wenigerkind H, Wanke R, Duchler Chiliad, Steinborn R, Mueller Chiliad, Brem GE: Adult cloning in cattle: potential of nuclei from a permanent cell line and from primary cultures. Mol Reprod Dev. 1999, 54: 264-272. x.1002/(SICI)1098-2795(199911)54:3<264::AID-MRD7>3.0.CO;2-Y.
-
Amano T, Kato Y, Tsunoda Y: Full-term development of enucleated mouse oocytes fused with embryonic stem cells from different prison cell lines. Reproduction. 2001, 121: 729-733. 10.1530/reprod/121.5.729.
-
Eggan Thou, Akutsu H, Loring J, Jackson-Grusby Fifty, Klemm One thousand, Rideout WM, Yanagimachi R, Jaenisch R: Hybrid vigor, fetal overgrowth, and viability of mice derived by nuclear cloning and tetraploid embryo complementation. Proc Natl Acad Sci Us. 2001, 98: 6209-6214. 10.1073/pnas.101118898.
-
Humpherys D, Eggan K, Akutsu H, Hochedlinger 1000, Rideout WM, Biniszkiewicz D, Yanagimachi R, Jaenisch R: Epigenetic instability in ES cells and cloned mice. Science. 2001, 293: 95-97. 10.1126/science.1061402.
-
Kato Y, Rideout Three West, Hilton K, Barton SC, Tsunoda Y, Surani MA: Developmental potential of mouse primordial germ cells. Evolution. 1999, 126: 1823-1832.
-
Wakayama T, Rodriguez I, Perry ACF, Yanagimachi R, Mombaerts P: Mice cloned from embryonic stalk cells. Proc Natl Acad Sci Us. 1999, 96: 14984-14989. 10.1073/pnas.96.26.14984.
-
Zhou Q, Jouneau A, Brochard V, Adenot P, Renard JP: Developmental potential of mouse embryos reconstructed from metaphase embryonic stalk cell nuclei. Biol Reprod. 2001, 65: 412-419.
-
Enright BP, Kubota C, Yang Ten, Tian 90: Epigenetic characteristics and evolution of embryos cloned from donor cells treated by Trichostatin A or v-aza-2'-deoxycytidine. Biol Reprod. 2003, 69: 896-903.
-
Jones KL, Hill J, Shin TY, Lui 50, Westhusin G: DNA hypomethylation of karyoplasts for bovine nuclear transplantation. Mol Reprod Dev. 2001, lx: 208-213. 10.1002/mrd.1079.
-
Zhou Q, Baquir S, Brochard 5, Smith LC, Renard JP: Donor nuclei are not well reprogrammed by nuclear transfer procedure. Biol Reprod. 2002, 66 (suppl 1): 237-238. (s345)
-
Cibelli P, Stice SL, Golueke PJ, Kane JJ, Jerry J, Blackwell C, deLeon FAP, Robl JM: Cloned transgenic calves produced from non-quiescent fetal fibroblasts. Science. 1998, 280: 1256-1258. x.1126/science.280.5367.1256.
-
Zakhartchenko V, Durcova-Hills G, Stojkovic One thousand, Schernthaner Westward, Prelle Chiliad, Steinborn R, Muller G, Brem One thousand, Wolf Eastward: Effects of serum starvation and re-cloning on the efficiency of nuclear transfer using bovine fetal fibroblasts. J Reprod Fertil. 1999, 115: 325-331.
-
Roh S, Shim H, Hwang WS, Yoon JT: In vitro development of green fluorescent poly peptide (GFP) transgenic bovine embryos after nuclear transfer using different cell cycles and passages of fetal fibroblasts. Reprod Fertil Dev. 2000, 12: i-6. 10.1071/RD00021.
-
Cho JK, Lee BC, Park JI, Lim JM, Shin SJ, Kim KY, Lee BD, Hwang WS: Development of bovine oocytes reconstructed with dissimilar donor somatic cells with or without serum starvation. Theriogenology. 2002, 57: 1819-1828. ten.1016/S0093-691X(01)00699-9.
-
Wakayama T, Yanagimachi R: Mouse cloning with nucleus donor cells of unlike age and type. Mol Reprod Dev. 2001, 58: 376-383. ten.1002/1098-2795(20010401)58:4<376::AID-MRD4>3.0.CO;2-Fifty.
-
Wakayama T, Perry Air conditioning, Zuccotti M, Johnson KR, Yanagimachi R: Total-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature. 1998, 394: 369-374. 10.1038/28615.
-
Forsberg EJ, Strelchenko NS, Augenstein ML, Betthauser JM, Childs LA, Eilertsen KJ, Enos JM, Forsythe TM, Golueke PJ, Koppang RW, Lange G, Lesmeister TL, Mallon KS, Mell GD, Misica PM, Pace MM, Pfister-Genskow M, Voelker GR, Watt SR, Bishop MD: Production of cloned cattle from in vitro systems. Biol Reprod. 2002, 67: 327-333.
-
Enright BP, Jeong BS, Yang X, Tian 90: Epigenetic Characteristics of Bovine Donor Cells for Nuclear Transfer: Levels of Histone Acetylation. Biol Reprod. 2003
-
Hirayu H, Dere WH: Rapoport B. Initiation of normal thyroid cells in main civilization associated with enhanced c-myc messenger ribonucleic acid levels. Endocrinology. 1987, 120: 924-928.
-
Baker TK, Carfagna MA, Gao H, Dow ER, Li Q, Searfoss GH, Ryan TP: Temporal gene expression analysis of monolayer cultured rat hepatocytes. Chem Res Toxicol. 2001, xiv: 1218-1231. 10.1021/tx015518a.
-
Hill JR, Winger QA, Burghardt RC, Westhusin ME: Bovine nuclear transfer embryo development using cells derived from a cloned fetus. Anim Reprod Sci. 2001, 67: 17-26. 10.1016/S0378-4320(01)00106-3.
-
Mayer West, Niveleau A, Walter J, Fundele R, Haaf T: Demethylation of the zygotic paternal genome. Nature. 2000, 403: 501-502.
-
Oswald J, Engemann South, Lane N, Mayer Westward, Olek A, Fundele R, Dean Westward, Reik Westward, Walter J: Agile demethylation of the paternal genome in the mouse zygote. Curr Biol. 2000, ten: 475-478. 10.1016/S0960-9822(00)00448-6.
-
Hoshikawa Y, Kwon HJ, Yoshida K, Horinouchi S, Beppu T: Trichostatin A induces morphological changes and gelsolin expression by inhibiting histone deacetylase in human carcinoma cell lines. Exp Jail cell Res. 1994, 214: 189-197. x.1006/excr.1994.1248.
-
Nakajima H, Kim YB, Terano H, Yoshida One thousand, Horinouchi S: FR90 a Strong Antitumor Antibiotic, Is a Novel Histone deacetylase inhibitor. Expt Cell Res. 1228, 241: 126-133. 10.1006/excr.1998.4027.
Acknowledgement
The authors would like to thank Marina Julian for careful reading and editing this manuscript.
Writer information
Authors and Affiliations
Corresponding writer
Authors' original submitted files for images
Rights and permissions
Virtually this article
Cite this article
Tian, Ten.C., Kubota, C., Enright, B. et al. Cloning animals past somatic cell nuclear transfer – biological factors. Reprod Biol Endocrinol 1, 98 (2003). https://doi.org/x.1186/1477-7827-1-98
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1186/1477-7827-one-98
Keywords
- nuclear transfer
- donor cell types
- donor age
- serum starvation
- prison cell passage
Source: https://rbej.biomedcentral.com/articles/10.1186/1477-7827-1-98
Posted by: blunthaideatel.blogspot.com

0 Response to "Which Statement Describes Cloning Animals By The Nuclear Transfer Technique?"
Post a Comment